Ted Ross Laboratory
-
Ted Ross Laboratory
- Principal Investigator
- Research
- Our Team
- Publications
- Careers
- Research News

Ted Ross, PhD
Global Director of Vaccine Development
Email: [email protected]
Location:
Cleveland Clinic Florida Research & Innovation Center
Research
The Ted Ross lab develops universal vaccines to target multiple strains, variants and types of viruses. Our goal with these vaccines is to increase the protection these vaccines provide, while decreasing the amount of booster shots required to maintain this protection. To this end, we have developed a novel technology called Computationally Optimized Broadly Reactive Antigen (COBRA) to create long-lasting universal vaccines that protect against the seasonal flu, animal-borne influenza (including swine and avian flu) and other respiratory viruses including COVID.
While we currently focus on influenza in all its forms, the lab has interest and expertise in vaccines against cancer; respiratory viruses including COVID and respiratory syncytial virus; HIV; and arboviruses.
For more about our approach to vaccine development, visit our Research tab.
Biography
Ted Ross, PhD, is the Global Director of Vaccine Development at Cleveland Clinic.
Dr. Ross joined Cleveland Clinic from the University of Georgia where he was the Georgia Research Alliance Eminent Scholar of Infectious Diseases and director of the Center for Vaccines and Immunology. Prior to that, he was director of Vaccines and Viral Immunity at the Vaccine and Gene Therapy Institute of Florida.
Dr. Ross has published more than 230 peer-reviewed papers and book chapters on infectious disease and vaccine development. He participates in several vaccine working groups, including at the NIH, the U.S. Centers for Disease Control and Prevention and the World Health Organization. He is an editorial board member of Vaccine. He was the President of the International Society for Vaccines (2020-2021).
Dr. Ross completed his undergraduate and graduate studies in zoology and microbiology at the University of Arkansas. He received his doctorate from Vanderbilt University and was awarded the inaugural Sidney P. Colowick Award in Outstanding Graduate Research. Dr. Ross performed post-doctoral fellowships at Duke University and at Emory University.
Education & Professional Highlights
Appointed
2022
Education & Fellowships
Fellowship - Emory University School of Medicine
Atlanta, GA USA
2000
Fellowship - Duke University School of Medicine
Durham, NC USA
1998
Graduate School - Vanderbilt University
Nashville, TN USA
1996
Graduate School - University of Arkansas, Fayetteville
Fayetteville, AR USA
1989
Undergraduate - University of Arkansas, Fayetteville
Zoology
Fayetteville, AR USA
1986
Research
Overview
As Global Director of Vaccine Research at Cleveland Clinic, Dr. Ross leads a multi-disciplinary team of scientists and clinicians in Ohio and Florida to design and test universal vaccines. Our team is focused on understanding how to elicit broadly reactive host responses against circulating and emerging pathogens with our next-generation vaccines.
Our vaccine research focuses on respiratory virus-induced diseases including influenza, COVID and respiratory syncytial virus (RSV). Our vaccine platforms also allow us to study HIV and arboviruses such as Dengue, Zika, Chikungunya and Rift Valley fever viruses.
A major focus of our research is developing a more advanced and longer-lasting flu vaccine to protect against multiple strains of the virus.
Universal Vaccine Research
Just like the organisms they infect, viruses are incredibly diverse. In fact, two of the “same” type of virus can differ so wildly that they need different treatments and vaccines. Just look at influenza. From seasonal sniffles that change so much every year they require annual booster shots, to animal-borne influenzas like swine flu or avian flu, each type of “flu” has its own unique signature in our bodies.
Current vaccines train our immune systems to recognize and destroy an individual signature unique to one viral strain. We have developed the technology to develop vaccines that protect against multiple strains of influenza, eliminating the need for multiple shots and annual boosters. Our technology is universal, letting us make combination COVID/flu vaccines. As we continue to refine our technology and expand our studies, we hope to expand beyond respiratory viruses and develop HIV vaccines, cancer vaccines and more.
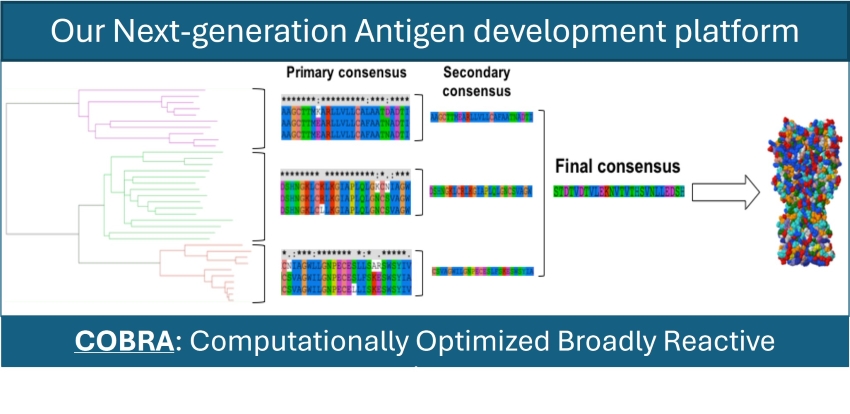
COBRA technology
Our team developed COBRA (Computationally Optimized Broadly Reactive Antigen) technology, a “consensus generation computational method” designed specifically to address diversity within and across related viruses, especially for influenza.
COBRA integrates thousands of diverse viral genetic and protein sequences collected from global surveillance efforts to identify conserved regions (consensus regions) across different strains or types of viruses. The more conserved a sequence is across highly diverse strains, the less likely that sequence is to mutate over time. For this reason, our influenza vaccines developed using COBRA protect against decades worth of past community isolates and are predicted to protect against decades’ worth of future influenza strains. This broad protection is designed to eliminate the need for a yearly influenza shot.
COBRA is especially versatile because it does not solely apply to one type of antigen. As long as a sequence is available, the technology can be applied to proteins and small peptides, mono- and polyclonal antibodies, and more.
In addition to protecting against respiratory viruses, COBRA vaccines have shown promise against HIV, arboviruses, and non-viral diseases like cancer.
Government Partnerships
The Ross Lab is partnered with government agencies from multiple countries to move our technology out of the lab to improve public health worldwide.
Dr. Ross is currently the Director of the Collaborative Influenza Vaccine Innovation Centers (CIVIC) network of the National Institutes of Health (NIH). He also an investigator in the INCENTIVE program with the European Union and the government of India.
Collaborate With Us
We are open and eager to collaborate with academic institutions and pharmaceutical companies alike. Please contact Dr. Ross at [email protected] with inquiries.
For fellow researchers: Our COBRA platform is highly diverse and adaptable, letting us design vaccines for virtually any disease. If you have resources and a preclinical model, we can work with you to design a vaccine for viruses, cancers, and more.
For pharmaceutical development: Our main goal is to deliver next-generation vaccines to as many people as possible, to improve public health in our community and beyond. We are always looking for platforms to expand our developmental capacity.
Our Team
Selected Publications
View publications for Ted Ross, PhD
(Disclaimer: This search is powered by PubMed, a service of the U.S. National Library of Medicine. PubMed is a third-party website with no affiliation with Cleveland Clinic.)
Careers
Please contact Dr. Ross at [email protected] for inquiries about job openings and mentorship opportunities!
Training at Lerner Research Institute
Our education and training programs offer hands-on experience at one of the nationʼs top hospitals. Travel, publish in high impact journals and collaborate with investigators to solve real-world biomedical research questions.
Learn MoreResearch News

Poor flu vaccine responses in people with immature and exhausted memory B cells may grant less effective immunity, but might be treated with improved vaccines.

The new Cleveland Clinic vaccine protects against lethal levels of multiple types of influenza including seasonal influenza, bird flu and other animal-borne flu viruses.

Elevated antibodies and immune cells associated with prior infection level out within six months of vaccination and need to be replenished with regular booster shots.

Next-generation vaccines are designed to protect against multiple viral strains and increase the time needed between booster shots.

In this newly created role, he will lead the development of novel vaccine platforms for a variety of infectious diseases, including influenza, HIV and COVID-19.